|
1910
|
1920
|
1930
|
1940
|
1950
|
1960
|
1970
|
1980
|
1990
|
2000
|
2010
|
2020
|
2040
Parker Hannifin History

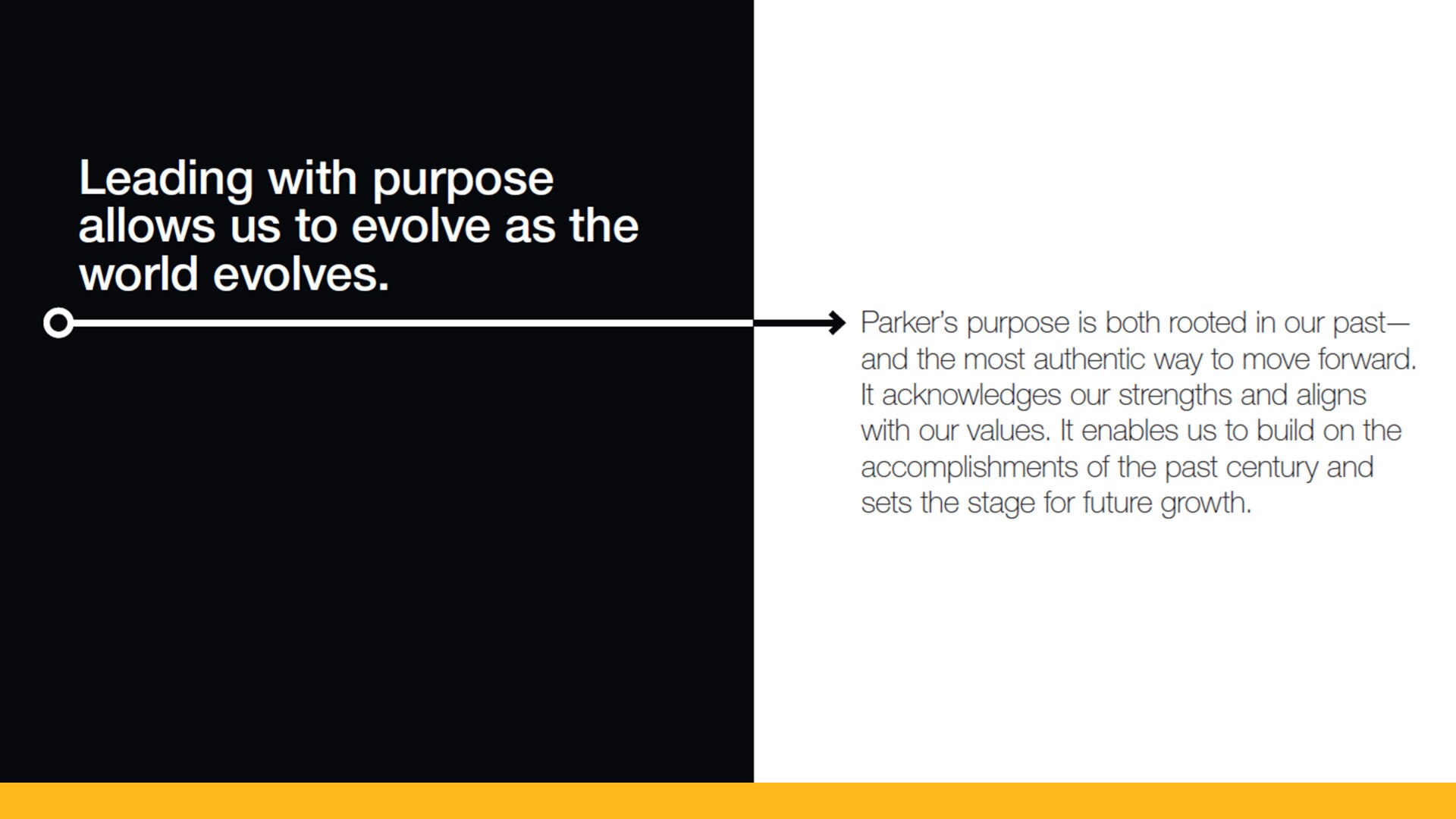
The Parker Journey
We invite you to take a journey with us. A journey begun with daring individual tenacity and shaped by remarkable collective accomplishments. A journey peppered with hardships and decorated with soaring successes. A journey from a two-person enterprise to one of the greatest companies in the world.
It’s the story of Parker.
Welcome.
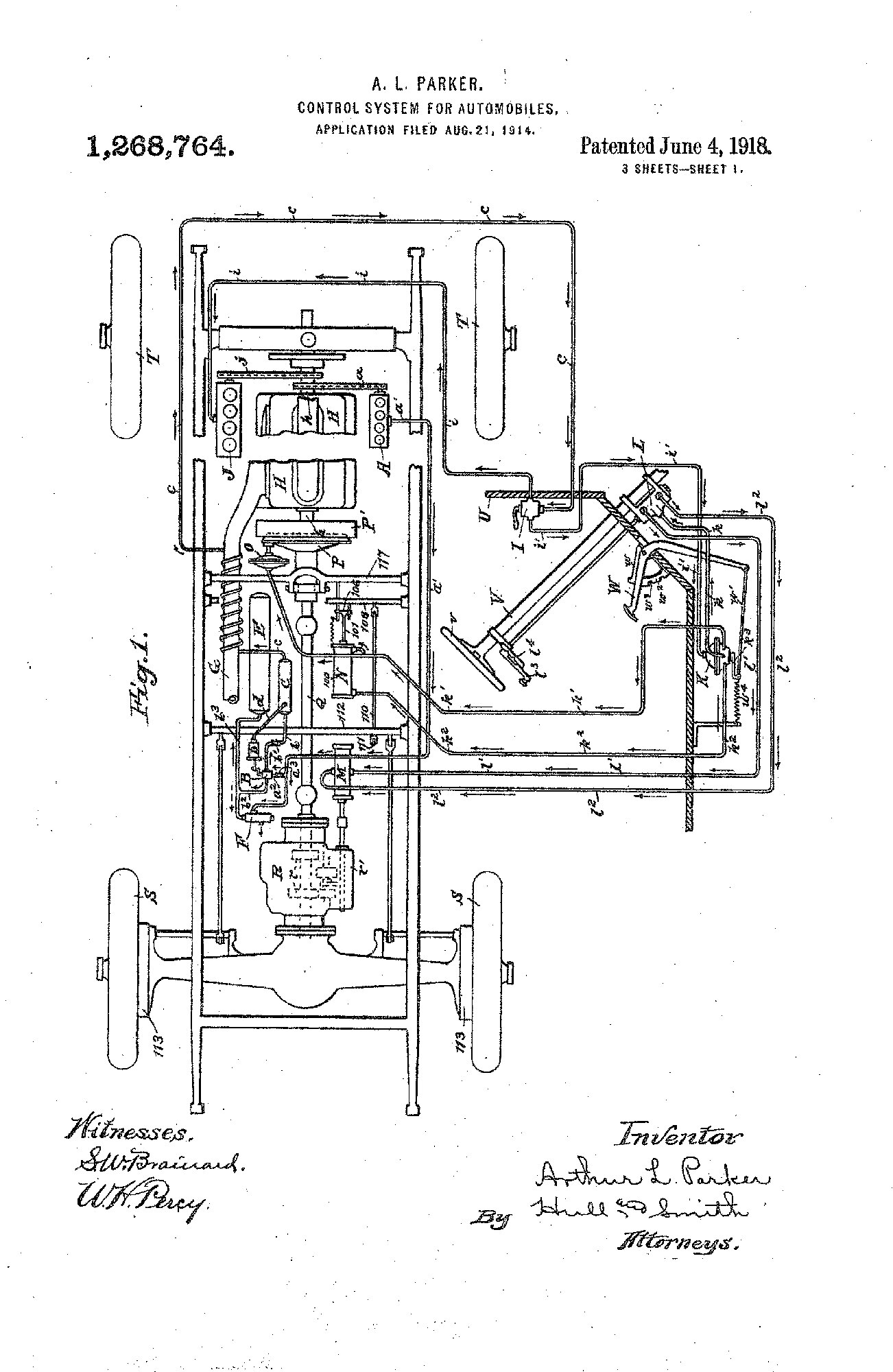
Parker Founded

1917
Birth of the Company
Traumatic Loss

1919
Traumatic Loss
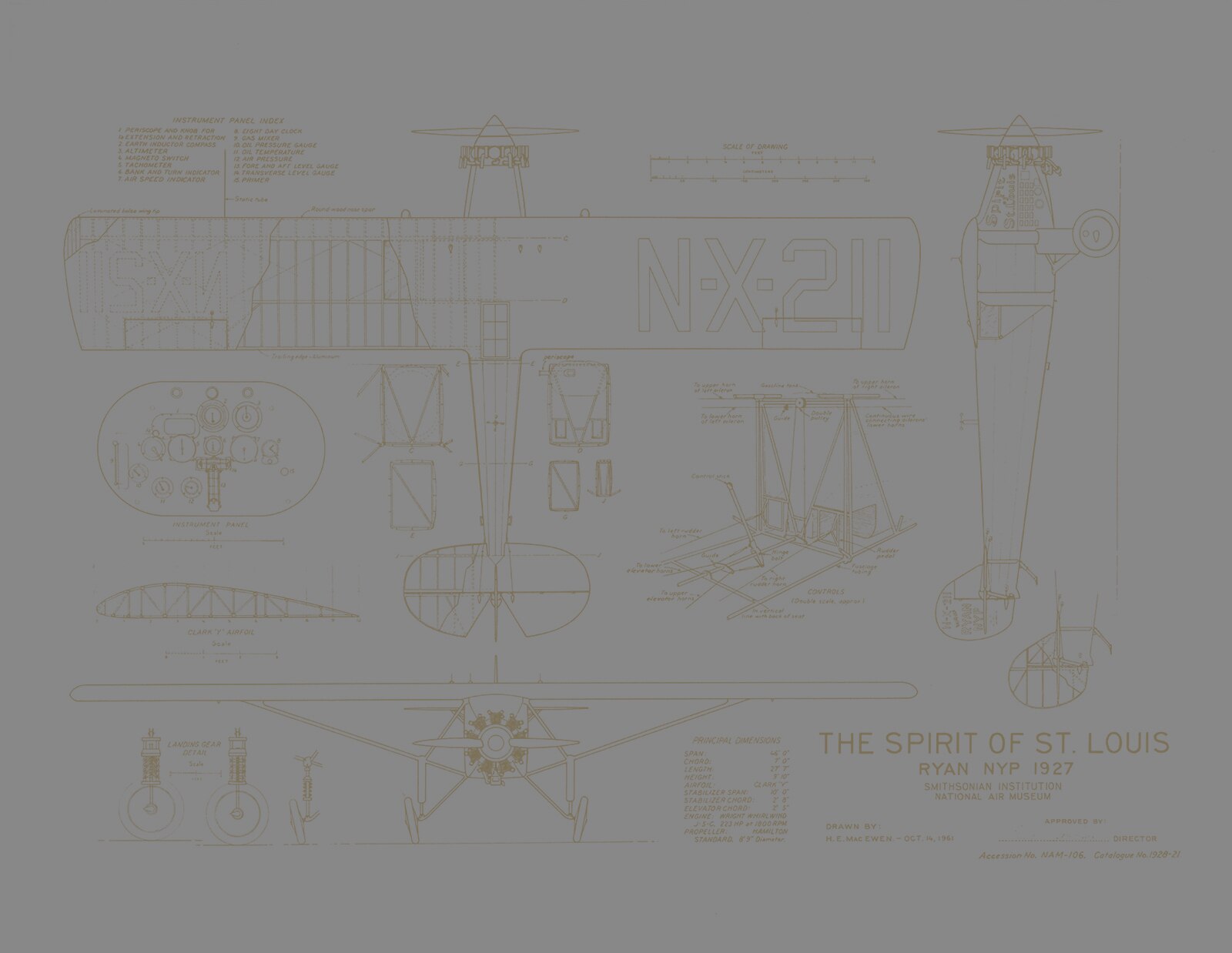
Crossing the Ocean

1927
Crossing the Ocean
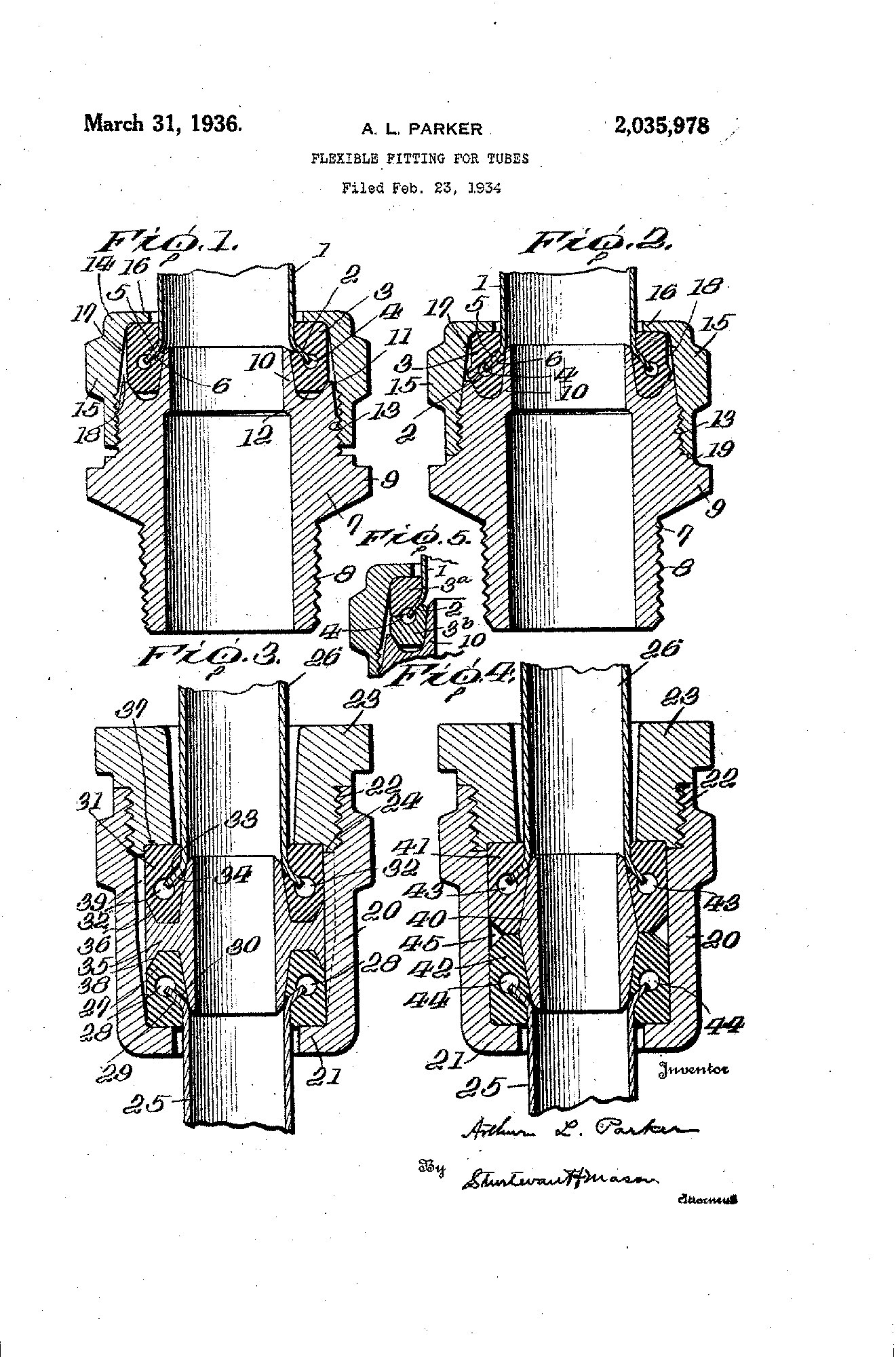
Early Expansion

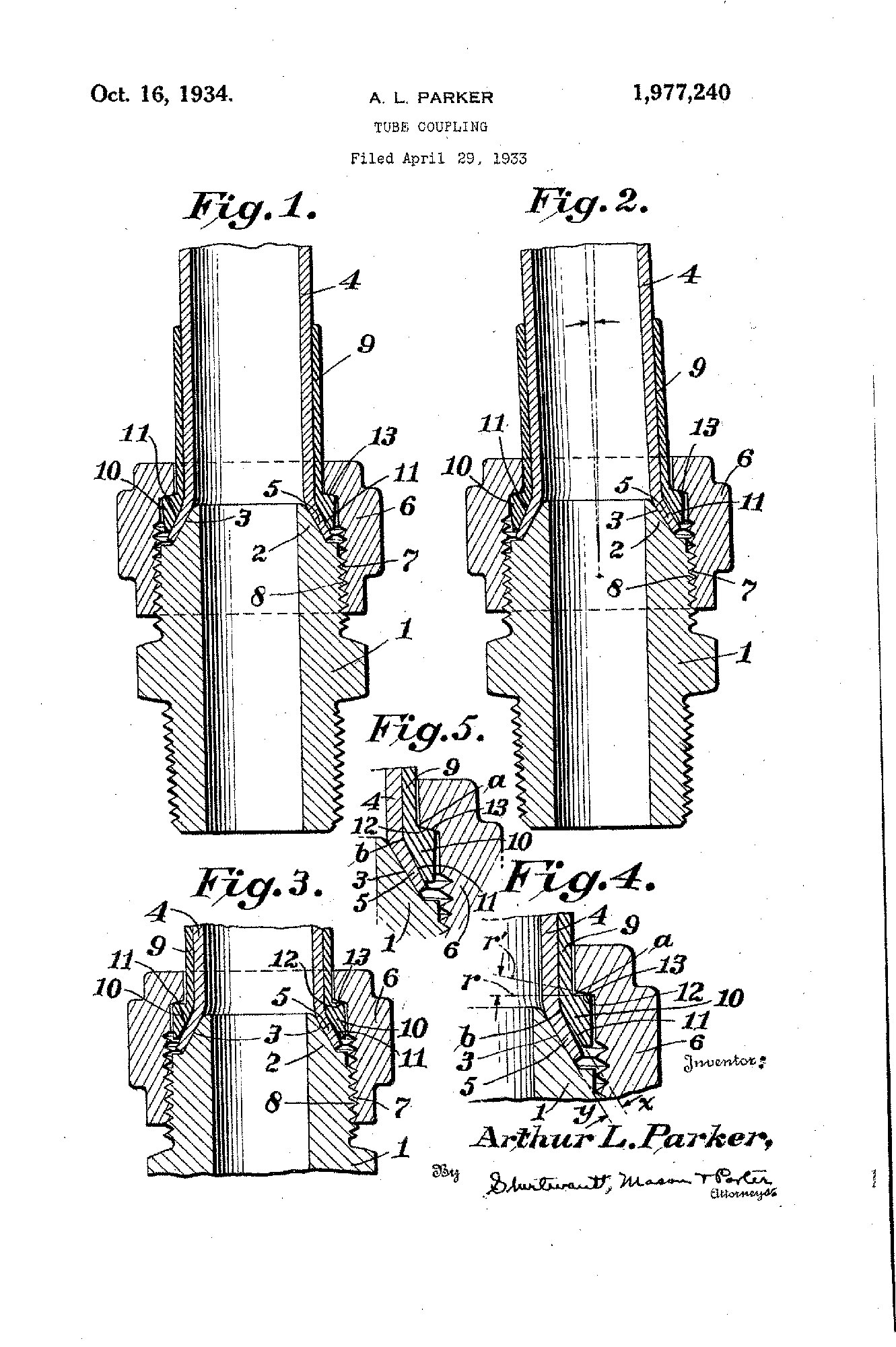
1934
Early Expansion
Winning the War

1939
Winning the War
End of an Era

1945
End of an Era
Becoming Parker Hannifin

1957
Becoming Parker Hannifin
On the Big Board

1964
On the Big Board
President Pat

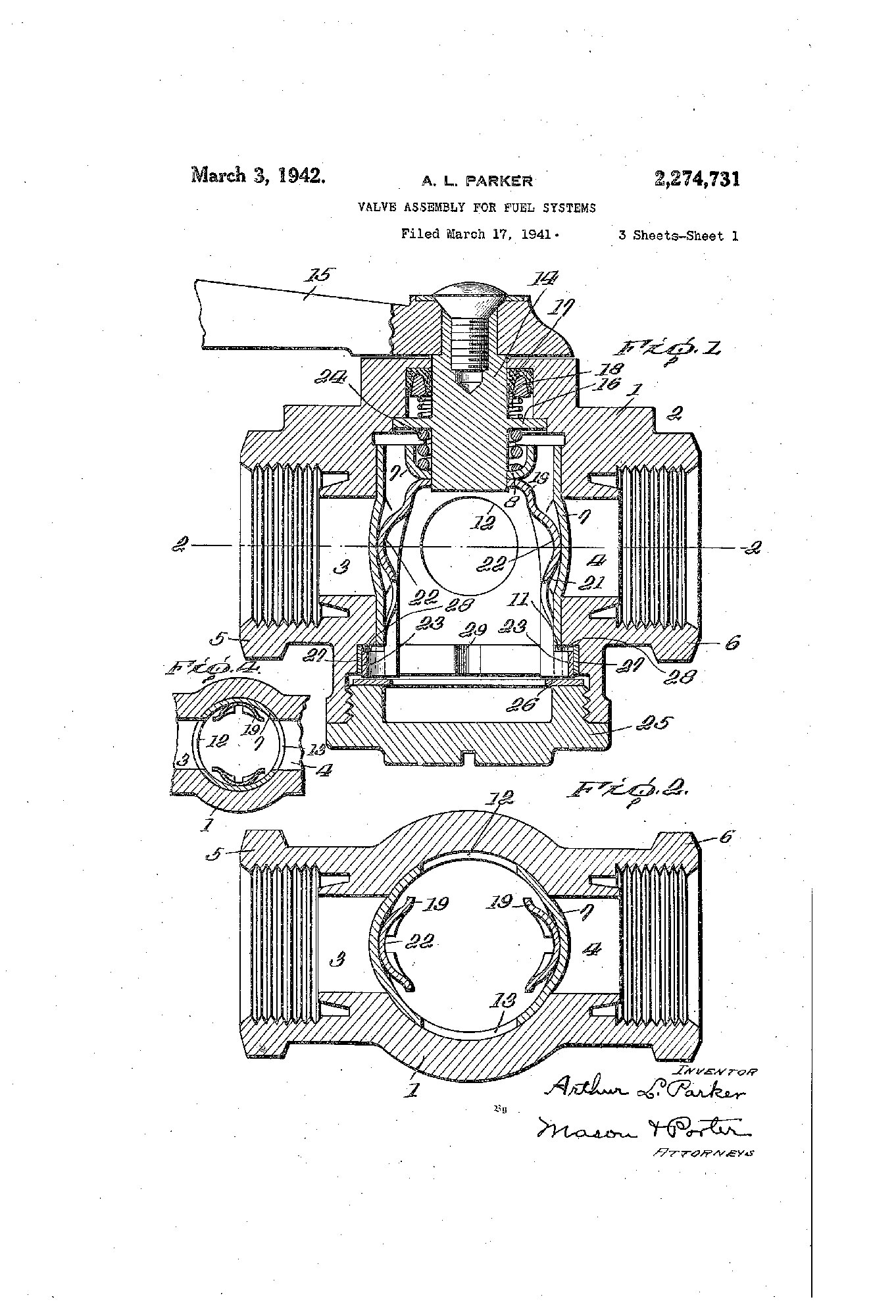
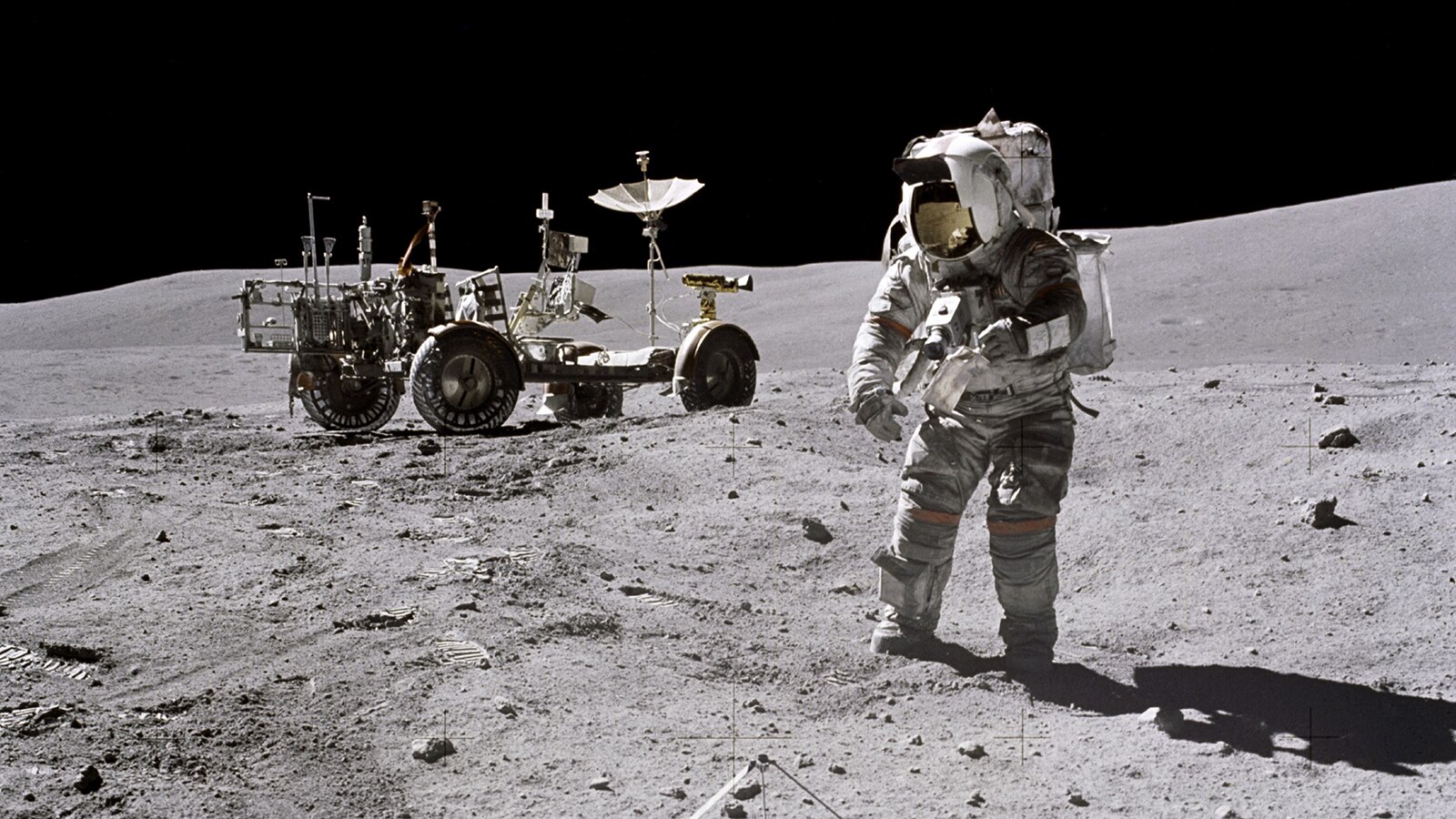
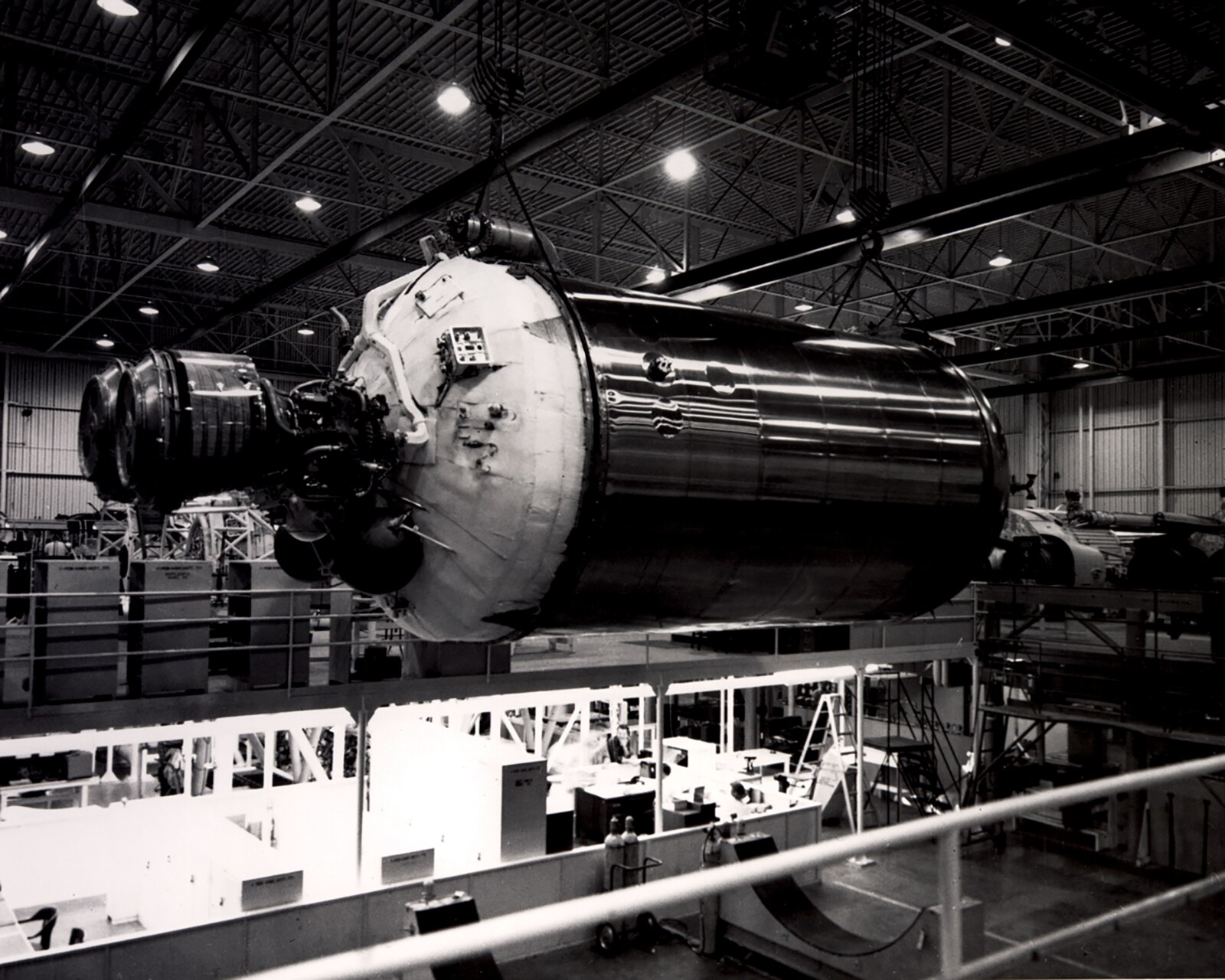
Parker in Space

1969
President Pat
Parker in Space
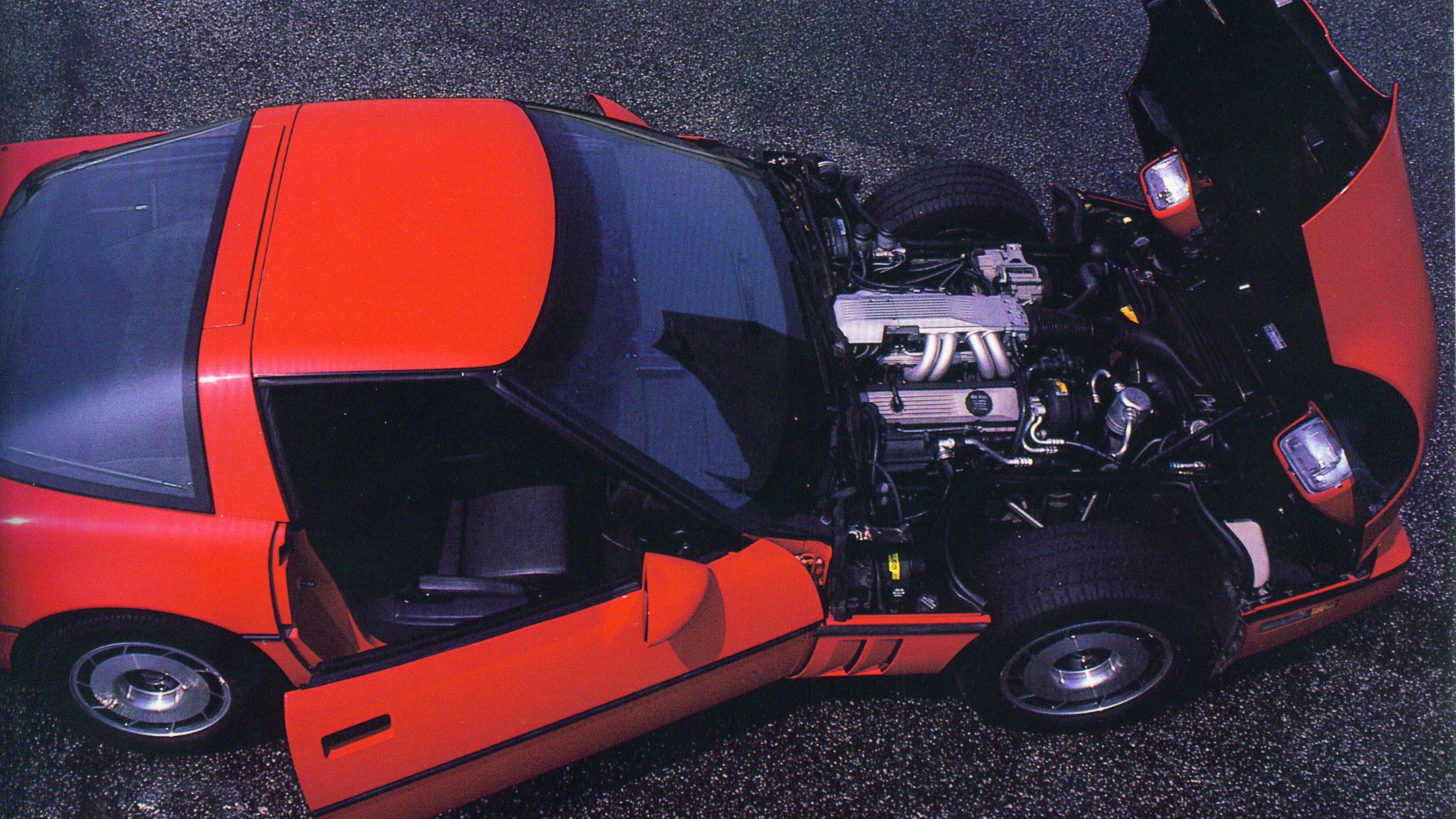
Sales Hit $1 Billion

1980
Global Player

1987
Innovative Business Model

1993
Innovative Business Model
New Global Headquarters

1997
New Global Headquarters
Sales Top $8 Billion

2000
Sales Top $8 Billion
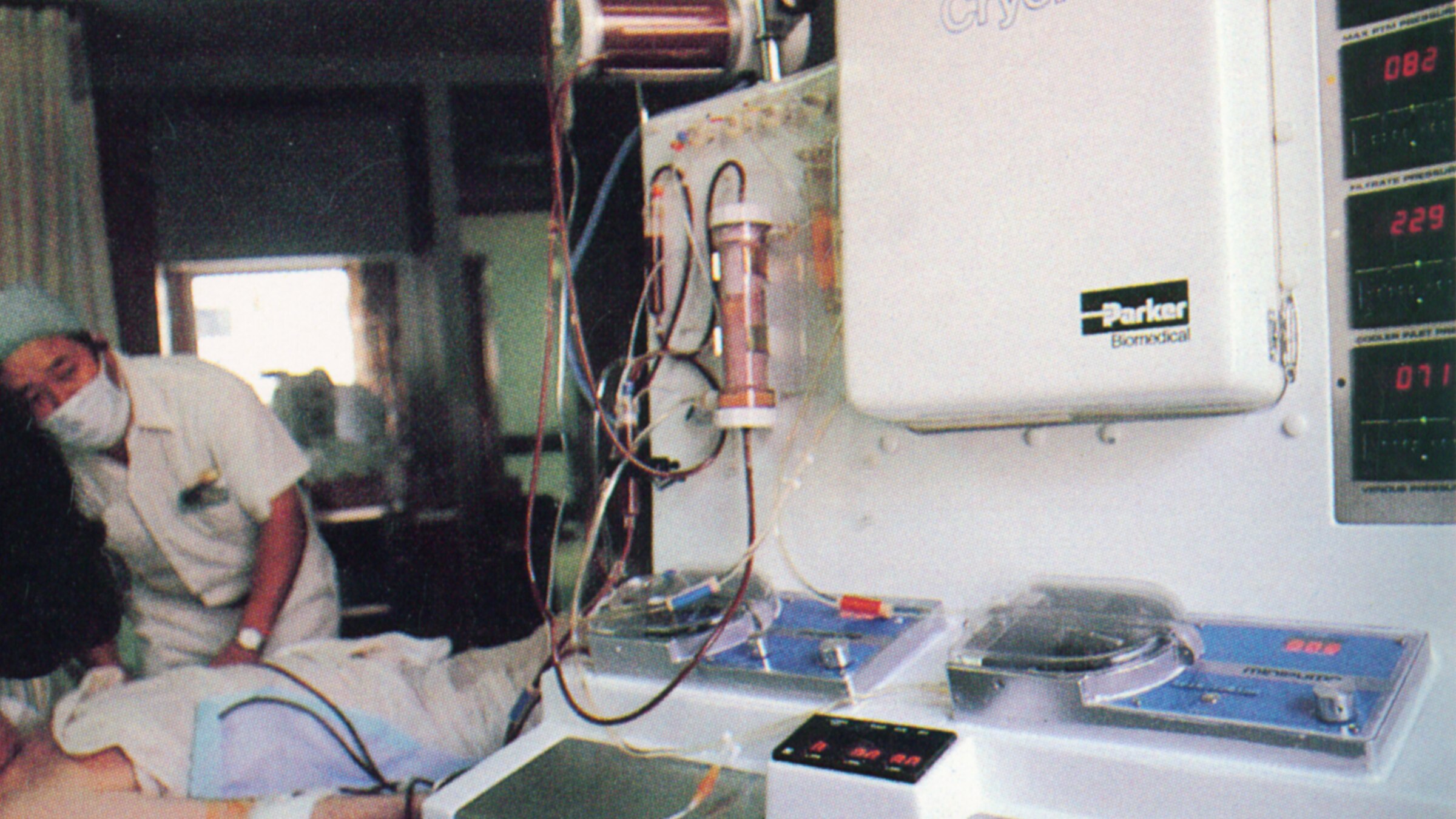
Helping a Nation Heal

2001
Helping a Nation Heal
Leadership Loss

2005
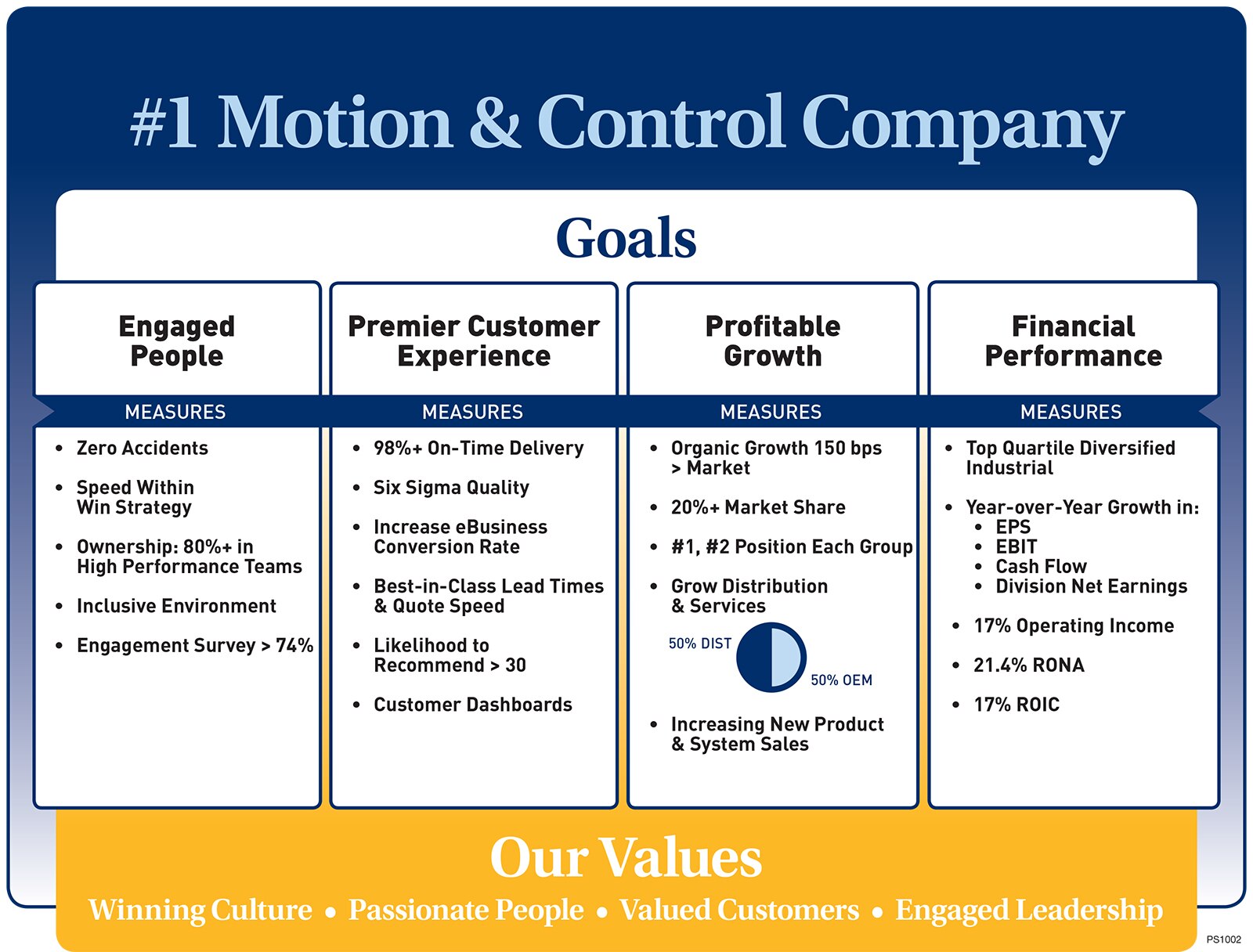
Winning Approach

2007
Winning Approach
Reorganized for Success

2013
Reorganized for Success
Winning Approach

2015
Winning Approach
Indego
Exoskeleton

2016
FDA Clears Indego Exoskeleton
The U.S. Food and Drug Administration (FDA) gives Parker clearance to market and sell the Indego® exoskeleton for clinical and personal use in the United States. Indego is a robotic exoskeleton or powered orthotic device that allows users to stand and walk and holds great promise for affording people with paraplegia a new level of independence.
The FDA’s clearance came following the completion of the largest exoskeleton clinical trial conducted to date in the U.S.
A Big Milestone

CLARCOR

2017
Parker Completes CLARCOR Acquisition
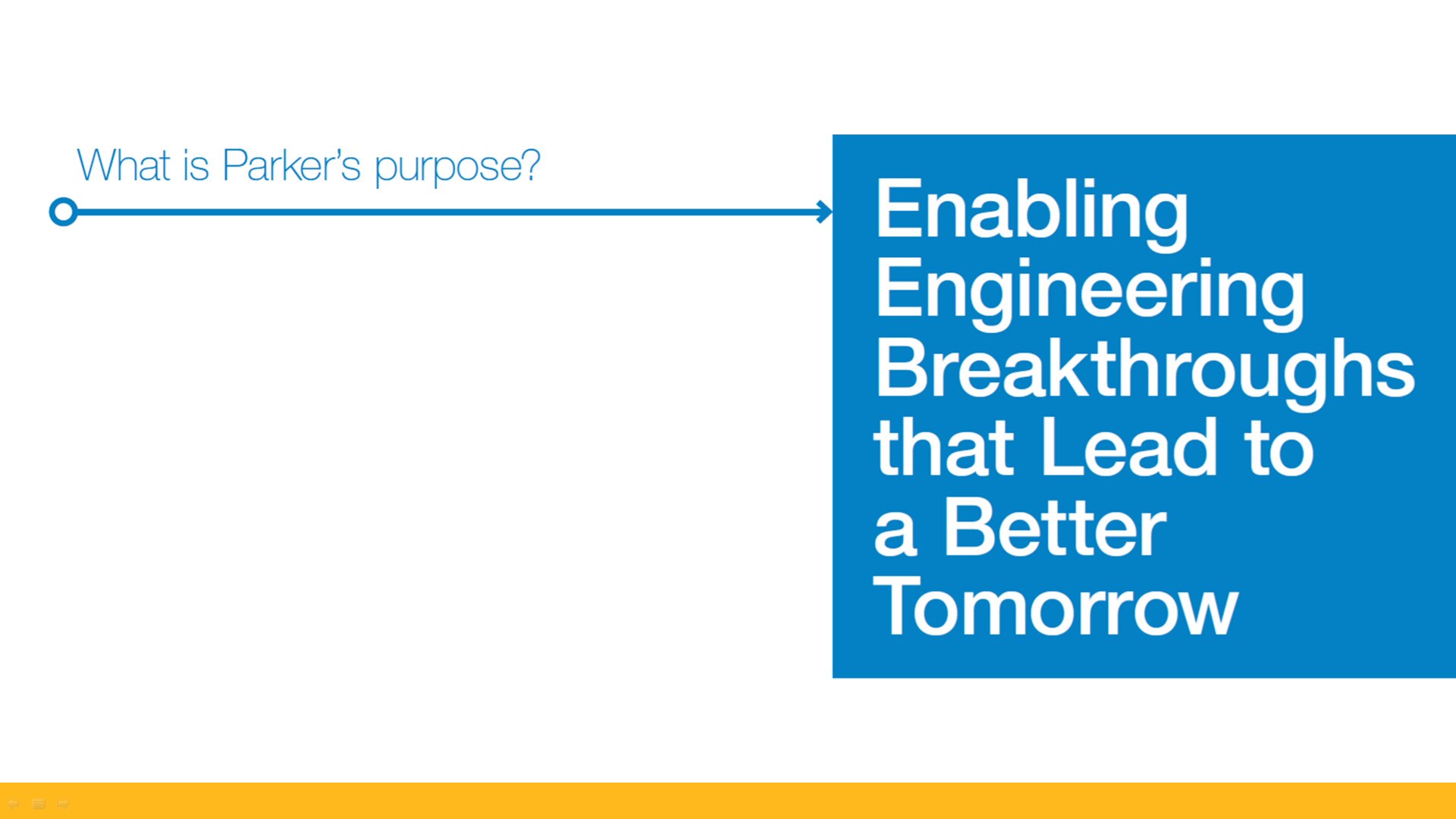
Defining
our Purpose

Exotic Metals

LORD

2019
Parker Completes Exotic Metals Acquisition
Parker Completes LORD Acquisition
Pandemic
Response

2020
Pandemic Response
Enabling a
Better Tomorrow

Meggitt

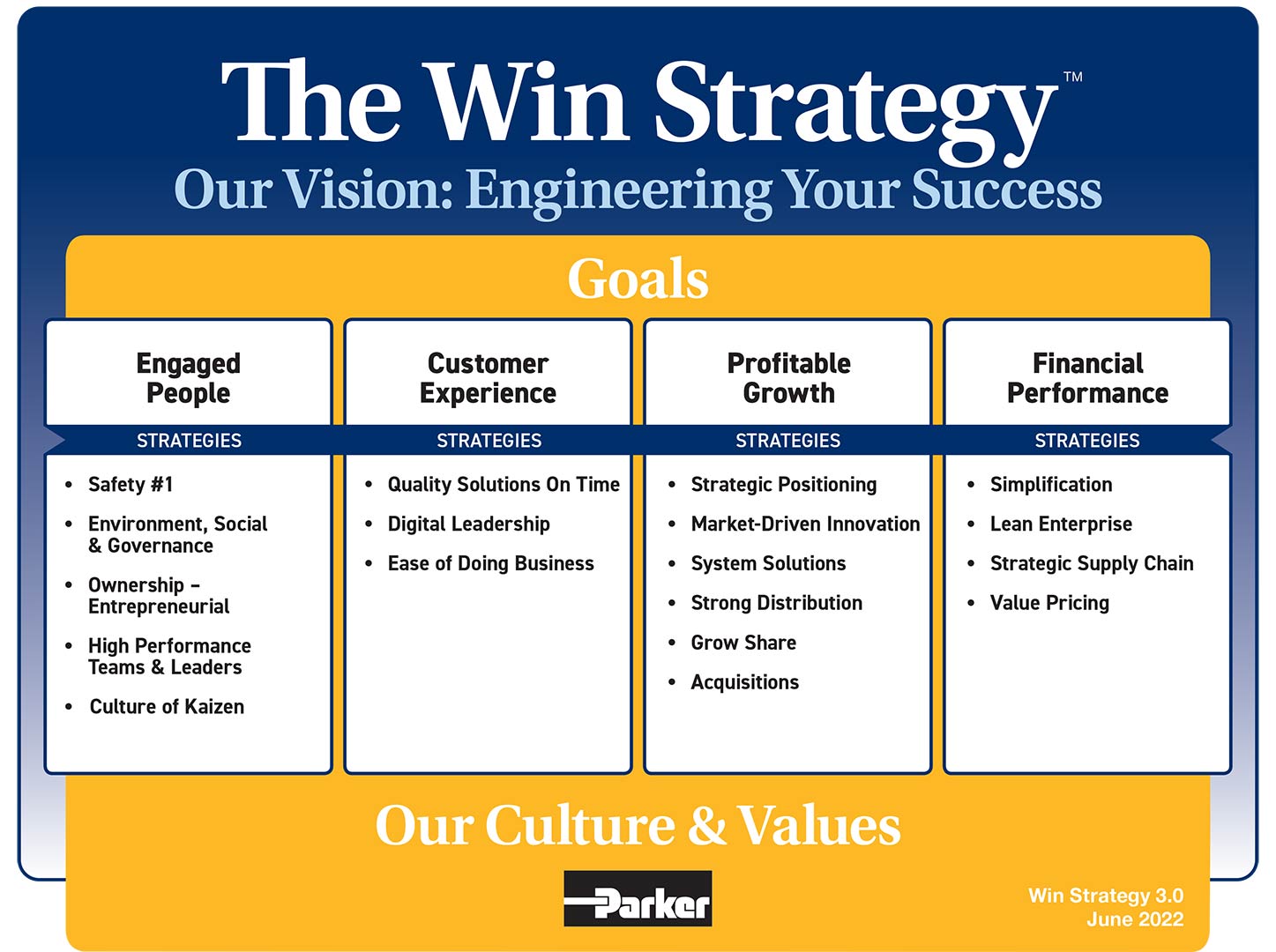
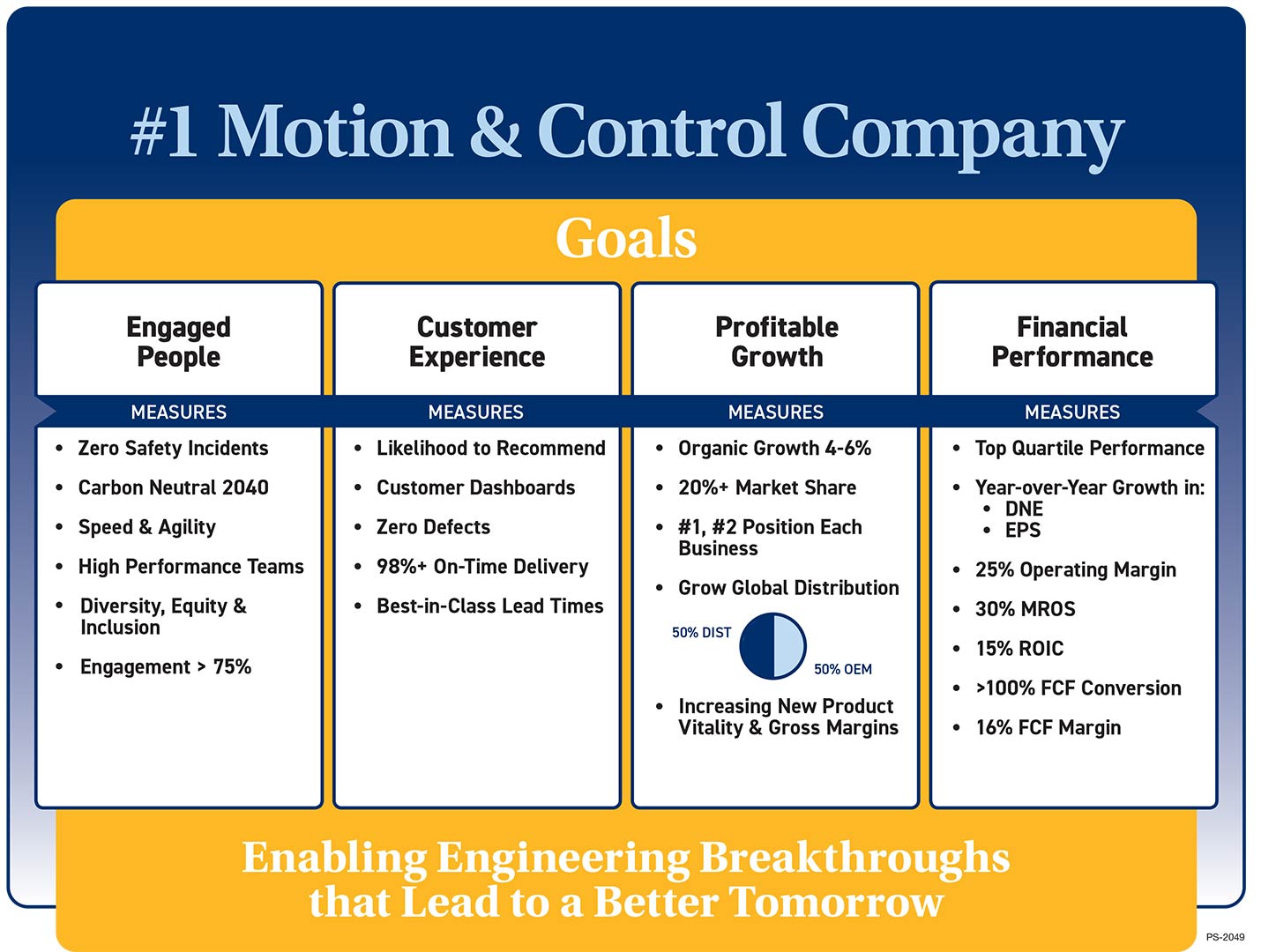
Win Strategy 3.0

2022
Parker Completes Acquisition of Meggitt PLC
Enabling a Better Tomorrow
New Leadership

2023
New Leadership
Seven BRGs

2024
Parker’s Seven BRGs
Parker’s Business Resource Groups (BRGs) are designed to enhance visibility, awareness and education for all team members. Each group serves as a platform for team members to connect, share experiences, and contribute to a vibrant workplace culture. All BRGs are open to all team members, and together, these seven BRGs will enrich our inclusive culture, ensuring every team member feels they belong, matter, and make a difference in creating a better tomorrow.