Preventing fire and or explosion of flammable materials and chemicals by excluding oxygen.
To support combustion or explosion, 3 critical components are required:
a) A fuel (something that burns or explodes in ambient air).
b) An ignition source such as a spark, static discharge, or naked flame for example.
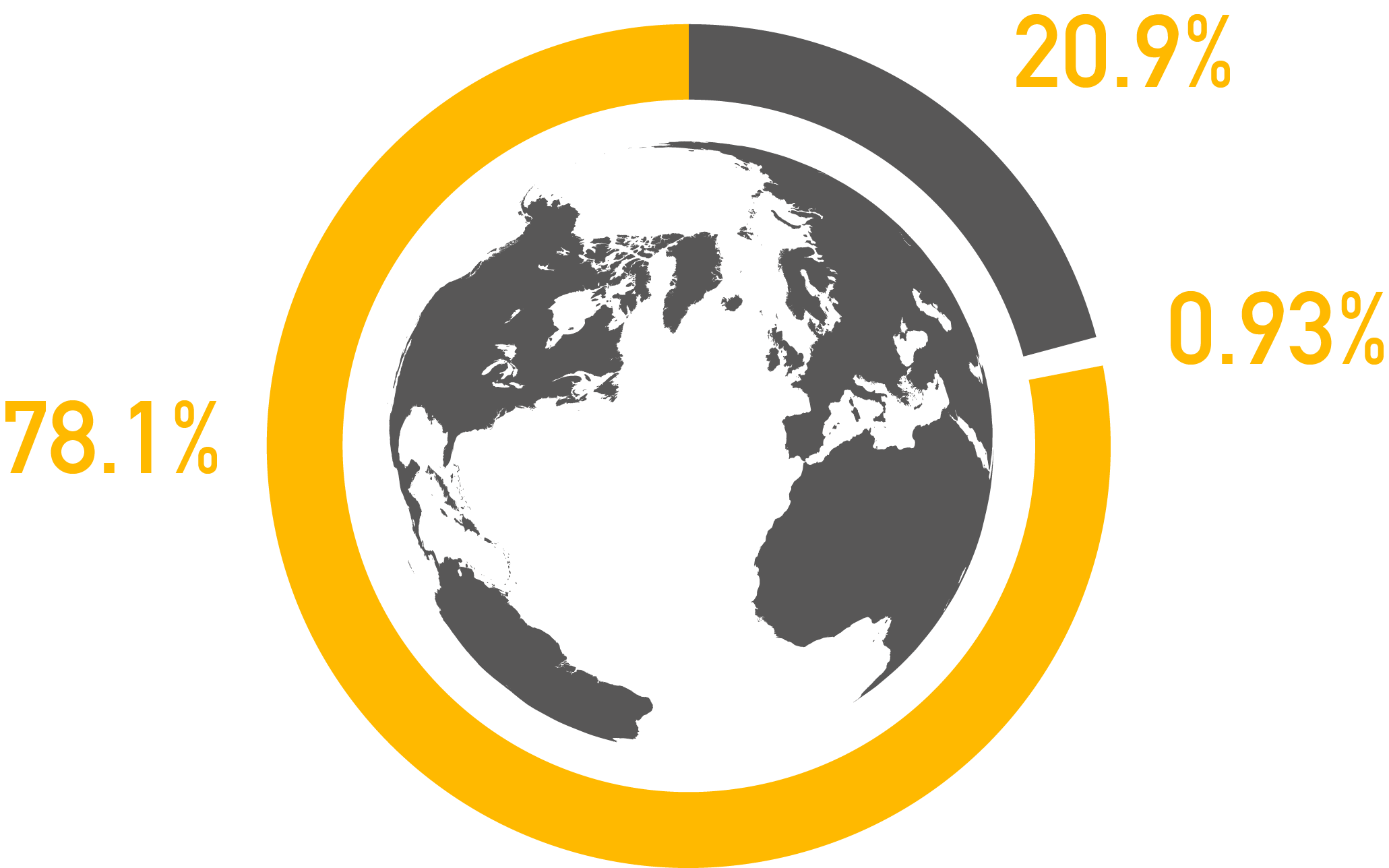
c) Oxygen – Generally most flammable things can burn or explode when the oxygen level is typically above 10%, so ambient air at nearly 21% supports combustion very well.
These 3 components are named the “Fire Triangle”. But like a triangle, if any one of the sides is removed, it collapses. In this case a collapse means a fire or explosion cannot happen. Displacing ambient air with nitrogen gas will remove the oxygen side of the triangle and prevent fire and or explosion.